The quality of our instruments as well as our excellent service is mainly responsible for our common success.
Our technical knowledge, the use of specialized machines with automatic production processes, constantly improving processes, our wide portfolio of more than 2500 products and steadily developed innovative instruments have been increasing us to one of the leading approved manufacturing enterprises in the dental sector in the world.
We are using unexceptional production materials that meet our particularly high-quality standards. Furthermore, these quality standards will be the focus of our daily activities in future.
All Carl Martin products fulfil the requirements of the EC-guideline 93/42/EWG and are marked with the CE-label.
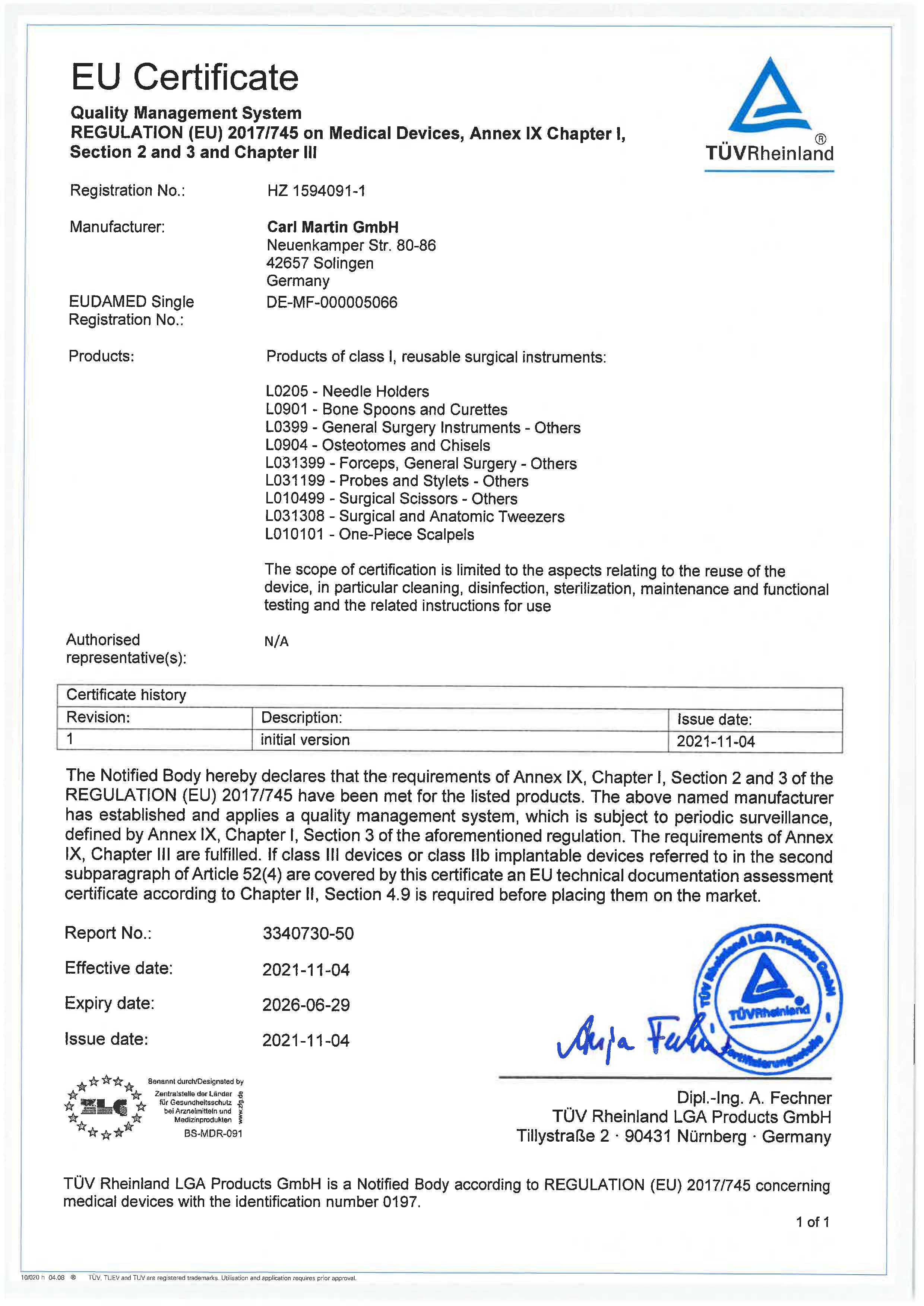
Since the introduction of our quality management system and the first certification according to DIN EN ISO 13485 by TÜV Rheinland in 1995, efficient structures and process-oriented workflows form the basis of a high-quality standard. The constant quality control as well as annual surveillance audits, guarantee the meeting of determined norms.
Currently, Carl Martin GmbH has a certified quality management system according to EN ISO 13485:2016.
Our risk management addresses to ISO 14971 and the application of the medical device law (MPG).
We are exclusively manufacturing instruments that are reusable and resterilizable. For all instrument groups, the proof as a reference-validation has been raised by the company ValiTech. Corresponding to our instruction for reprocessing QSA 313 according to DIN EN ISO 17664 our instruments can be processed with a standard thermal cleaning and disinfection process according to DIN EN ISO 15883.
- Quality Management
- Contact person
- Social Engagement
Quality Management
Our website uses cookies to ensure the best possible presentation and handling.
In addition, we evaluate anonymized data on visitor activity in order to optimize our website.
In addition, we evaluate anonymized data on visitor activity in order to optimize our website.